Popular

TPI.tv videos
InnovationPolicyBeginner
TPI.tv: improving science through animal-free innovations and research
Introducing TPI.tv : a video platform by experts striving to improve science through animal-free innovations and research.

Innovation examples
HealthToxicologyIn vitro
Assessing respiratory toxicity using in vitro models
The airways form a barrier for inhaled compounds, however, such compounds may cause local effects in the airways or may lead to lung diseases, such as fibrosis or COPD. Cell models of the respiratory tract, cultured at the air-liquid-interface (ALI) are a relevant model to assess the effects of inhaled compounds on the airways. Such models allow human relevant exposure, which is via the air, and assessment of effects on the epithelial cell layer. At RIVM we use air-liquid-interface cultured cell models and expose these to airborne compounds to assess the effects of agents such as nanomaterials, air pollutants or compounds from cigarette smoke. By using a mechanism-based approach to assess the effects of these compounds we invest in animal-free alternatives that better predict adverse effects in humans.

Innovation examples
HealthToxicology
Zebrafish in toxicity testing
Zebrafish are increasingly recognised as a useful model for toxicity testing of chemical substances. Testing strategies are becoming more based on mechanisms of toxicity structured in adverse outcome pathways describing the chain of events leading to toxicity or disease. Using a battery of dedicated in vitro and in silico assays, insight can be gained in how exposure leads to disease. For certain diseases it is known that toxicity relies on the interaction between different organs and cell types, which requires research on whole organisms in addition to simple in vitro models. The zebrafish is considered a valuable whole organism model in a mechanism-based testing strategy. At RIVM, the zebrafish embryo model is used for testing the effect of chemical substances on several adverse outcomes and diseases.
For more information see: https://ehp.niehs.nih.gov/doi/10.1289/EHP9888; https://doi.org/10.3390/ijerph18136717; www.linkedin.com/in/harm-heusinkveld

Innovation examples
ToxicologyIn vitroOrgan-on-Chip
Cartilage-on-a-chip for studying joint degenerative diseases
Carlo Alberto Paggi is currently a PhD candidate at the University of Twente in the research group of Prof. Marcel Karperien and Prof. Séverine Le Gac. Karperien’s lab focus on the biological aspects of osteoarthritic research while Le Gac’s specialize in organ-on-chip development. The project of Carlo Alberto is developing a joint-on-chip platform to create a reliable in vitro model to study disease progression in osteo- or rheumatoid arthritis. The model combines different organ-on-chips aimed at replicating each a tissue around the joint such as cartilage, bone and ligaments. This new technology focuses on better reproducing human models and at substituting the use of animal models for drug research. If you want to know something more about the project and the groups, you can follow the link in the video.
Carlo Paggi was nominated for the Hugo van Poelgeest prize for his research on a cartilage-on-a-chip model to study joint degenerative diseases
Karperien’s lab of Developmental Bioengineering: https://www.utwente.nl/en/tnw/dbe/
Le Gac’s lab of Applied Microfluidics for BioEngineering Research: http://www.severinelegac.com/
Linkedin: https://www.linkedin.com/in/carlo-alberto-paggi-76500b135/

Questions
HelpathonsHealth
Helpathon #4 - can you help Frank?
Can you help Frank with integrating an immune system into a macaque lung organoid to address local immunity to tuberculosis with his vaccination strategy?
Join Helpathon #4, look at www.tpihelpathon.nl/coming-up !
Frank Verreck does research on tuberculosis at the Biomedical Primate Research Center (BPRC). Tuberculosis is the most deadly infectious disease worldwide! For the past hundred years, BCG (Bacillus Calmette Guérin) vaccinations take place through the skin. Research shows that macaques can be better protected from this infection by vaccination through their lungs. Frank really wants to further study the potential of this alternative vaccination strategy. He wants to understand how this BCG vaccination works in macaques lungs.

Innovation examples
In vitroOrgan-on-Chip
Unified organoid system for modeling heart and kidney interaction on-a-chip
Beatrice Gabbin is a PhD candidate at the Anatomy and Embryology Department of the Leiden University Medical Center. Her project is shared with the Nephrology Department and focusses on the study of the cardiorenal axis in vitro. Both heart and kidneys have vital functions in the human body and reciprocally influence each other’s behavior: pathological changes in one can damage the other. There are already multiple independent in vitro (human) models of heart and kidney, but none have so far captured their dynamic crosstalk. The aim of the project is therefore to develop a microfluidic system which can be used to study heart and kidney interaction in vitro. For this purpose, cardiac microtissues and kidney organoids derived from human induced pluripotent stem cells are generated and loaded onto a 3D perfusion chip for their dynamic co-culture. This system enables the study the cardiac and kidney interaction with a high level of control. The validation of a unified organoid system will enable the investigation of diseases involving the two organs and their potential treatments. Read more via the link in the video and https://doi.org/10.1016/j.mtbio.2023.100818.
Innovation examples
HealthIn vitroOrgan-on-Chip
Stem cell derived Vessels-on-Chip to study brain disorders
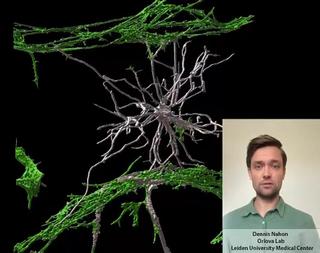
Dennis Nahon is a PhD candidate in the Department of Anatomy and Embryology at the Leiden University Medical Center. In his research, under supervision of Dr. Valeria Orlova (https://www.orlovalab.com/) and Prof. Dr. Christine Mummery, he aims to mimic a blood vessel in the brain by combining different stem cell derived cell types, in a 3D Vessel-on-Chip model. Here, an example of these in vitro blood vessels is shown in which certain brain cells known as astrocytes (in white) interact with the blood vessels (in red). This model paves the way for investigating brain vessels outside the human body, while reducing the need for animal models.

Projects and initiatives
HealthInnovationPolicyBeginner
We all want a safer world for humanity, animals and the environment: Transition Animal-free Innovation
Why is the transition to animal-free research so important? What are animal-free models? How does TPI (Transition Animal-Free Innovation) encourage their development and use? And who are we working with to make this happen? We explain this in our animation.
More and more animal-free tests and research methods are becoming available, but not all research questions or safety tests can be answered in this way yet. In addition, the validation, qualification and acceptance of non-animal innovations still lags behind. Therefore, the Dutch Ministry of Agriculture, Nature and Food Quality (LNV) stimulates the development and application of animal-free innovations. This is done with the partner programme Transition Animal-free Innovation (TPI).

Innovation examples
HealthToxicologyIn silico
AI agents for safer science: How AI is Changing Chemical Risk Assessment
This video introduces a novel approach to chemical safety, where intelligent digital agents guided by large language models support scientists in making faster, more transparent decisions. By automating complex workflows and integrating tools like the OECD QSAR Toolbox, these agentic systems help prioritise research, reduce reliance on animal testing, and pave the way for safer, more sustainable innovation.

Innovation examples
HealthToxicologyIn silico
Predictive computer models for protein binding
In this video Linde Schoenmaker (Leiden University) explains how she and her colleagues are making computer models to predict the safety of new chemicals within the VHP4Safety project.

Expert interviews
Policy
Charlotte Blattner, Harvard Law School: Transition needs community efforts
Charlotte Blattner is a visiting researcher at Harvard Law School on the Animal Law & Policy Program and explains that a transition is needed to move away from animal testing. This transition needs to be a just transition, a community effort where all stakeholders are involved to replace animal testing for animal-free innovations.

Expert interviews
HealthToxicologyIn vitro
Erwin Roggen, ToxGen Solutions: Applying animal free testing approaches
Erwin Roggen explains his role as pioneer in the development of technologies for animal-free application. His product, ToxGenSolutions, provides test methods required for modern testing and assessment of compounds and products. It builds on a virtual generic platform of leading test and technology developers providing novel technologies addressing key events in adverse outcome pathways.