Popular

Innovation examples
HealthToxicology
Zebrafish in toxicity testing
Zebrafish are increasingly recognised as a useful model for toxicity testing of chemical substances. Testing strategies are becoming more based on mechanisms of toxicity structured in adverse outcome pathways describing the chain of events leading to toxicity or disease. Using a battery of dedicated in vitro and in silico assays, insight can be gained in how exposure leads to disease. For certain diseases it is known that toxicity relies on the interaction between different organs and cell types, which requires research on whole organisms in addition to simple in vitro models. The zebrafish is considered a valuable whole organism model in a mechanism-based testing strategy. At RIVM, the zebrafish embryo model is used for testing the effect of chemical substances on several adverse outcomes and diseases.
For more information see: https://ehp.niehs.nih.gov/doi/10.1289/EHP9888; https://doi.org/10.3390/ijerph18136717; www.linkedin.com/in/harm-heusinkveld

Innovation examples
HealthIn vitroOrgan-on-Chip
An iPSC-derived blood-brain barrier to model neurodegeneration
The blood-brain barrier is a layer of cells that protects our brain from harmful compounds. However, due to this tight barrier, many drugs to treat neurological diseases cannot enter the brain either.
There are currently no good models to test these types of drugs. Henrique Nogueira Pinto is a PhD candidate at the Vrije Universiteit in Amsterdam. He is developing a blood-brain barrier model coupled to mini-brains. With this model, he aims to more reliably test how drugs can be transported over the blood-brain barrier and what their effect on the brain is.
Click on the info button for the full version of the video. Click here (https://fluidsbarrierscns.biomedcentral.com/articles/10.1186/s12987-022-00316-0#Sec3) for a review of the current status of in vitro models for the blood-brain barrier.

Questions
HelpathonsHealth
Helpathon #4 - can you help Frank?
Can you help Frank with integrating an immune system into a macaque lung organoid to address local immunity to tuberculosis with his vaccination strategy?
Join Helpathon #4, look at www.tpihelpathon.nl/coming-up !
Frank Verreck does research on tuberculosis at the Biomedical Primate Research Center (BPRC). Tuberculosis is the most deadly infectious disease worldwide! For the past hundred years, BCG (Bacillus Calmette Guérin) vaccinations take place through the skin. Research shows that macaques can be better protected from this infection by vaccination through their lungs. Frank really wants to further study the potential of this alternative vaccination strategy. He wants to understand how this BCG vaccination works in macaques lungs.
Innovation examples
HealthIn vitroOrgan-on-Chip
Stem cell derived Vessels-on-Chip to study brain disorders
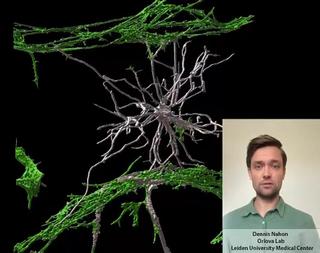
Dennis Nahon is a PhD candidate in the Department of Anatomy and Embryology at the Leiden University Medical Center. In his research, under supervision of Dr. Valeria Orlova (https://www.orlovalab.com/) and Prof. Dr. Christine Mummery, he aims to mimic a blood vessel in the brain by combining different stem cell derived cell types, in a 3D Vessel-on-Chip model. Here, an example of these in vitro blood vessels is shown in which certain brain cells known as astrocytes (in white) interact with the blood vessels (in red). This model paves the way for investigating brain vessels outside the human body, while reducing the need for animal models.
New

Questions
HelpathonsPolicyBeginner
Helpathon #12 – Can you help Erica?
We are inviting Dutch-speaking citizens from all walks of life to join a unique Helpathon and help Erica van Oort, coordinator of the Animal-Free Transition Program (TPI) in the Netherlands. No prior knowledge of animal testing is required—your fresh perspective can help Erica communicate more effectively about animal-free research.
We strongly believe that well-informed citizens are key to improving democratic policy-making on health research, with and without animals. Please share this invitation to at least one suitable person who could contribute—and of course, you are warmly welcome to join as well.

Projects and initiatives
HealthToxicology
The NAM Navigator: A unique repository for information on the validation and acceptance of New Approach Methodologies
The NAM navigator is an innovative knowledge portal to navigate you to and through valuable information on the development, standardization, validation and acceptance of New Approach Methodologies (NAM). The NAM Navigator acts as an online guide that provides specific information needed in each of these steps, thereby increasing the broad use of animal-free innovations. Follow the link in the video to start navigating!

Projects and initiatives
HealthToxicologyIn vitroIn silico
VHP4Safety project
The safety testing of chemicals and pharmaceuticals traditionally relies on animal studies. However, these raise ethical concerns and often fail to accurately predict human responses. New scientific developments offer opportunities to build a Virtual Human Platform (VHP) for safety assessment, a platform that enables assessment based solely on human physiology and biology, integrating data from in vitro and in silico models. This video explains how we are developing the VHP through an interdisciplinary approach. Read the paper in the videolink or visit or VHP4Safety (https://vhp4safety.nl/) for more information.

Innovation examples
HealthToxicologyIn silico
AI agents for safer science: How AI is Changing Chemical Risk Assessment
This video introduces a novel approach to chemical safety, where intelligent digital agents guided by large language models support scientists in making faster, more transparent decisions. By automating complex workflows and integrating tools like the OECD QSAR Toolbox, these agentic systems help prioritise research, reduce reliance on animal testing, and pave the way for safer, more sustainable innovation.